SOIL ORGANIC MATTER
Soil organic matter is an accumulation of plant and animal residue in varying stages of decay. These materials are continually being broken down by soil microorganism. Consequently, organic matter is a rather transitory soil component, and must be renewed constantly through the addition of new plant or animal residues.
Even though the percentage of organic matter in soil is relatively small, normally varying from 2% to 6% by weight, its influence on soil properties and plant growth is far greater than the low percentage would indicate.
The following are ways in which organic matter functions in soils:- As a granulator of mineral particles.
- As a major source of the mineral elements phosphorus, sulfur and nitrogen.
- Increases water holding capacity.
- As the main source of energy for soil organisms.
The more soluble organic substances, such as sugars and starches, are utilized rapidly. Less soluble organic substances, such as the natural carbohydrate hemicelluloses and lignins, are decomposed more slowly.
Soil organic matter consists of two general groups:- Original tissue (decomposed roots and tops of plants, and animal remains).
- Humus (decomposed roots and tops of plants and animal remains).
Humus is usually black or brown in color, and is colloidal in nature (Colloidal means it will stay suspended in soil solutions). Its capacity to hold water and nutrients far exeeds that of clay, its inorganic counterpart. Thus, it takes only small amount of humus to greatly enhance the soil's water holding and plant production capacities.
Soil Water
Water is held within the soil pores with varying degrees of tenacity, depending on the amount of water present, and the size of the pores. Soil solution, which is very important as a medium for supplying nutrients to the plants, is made up of soil water and dissolved nutrients. There are three basic forms of soil moisture:- Hygroscopic: A thin layer of moisture surrounds the soil particles; there is a very strong attraction between the soil particles and the water molecules, and if the film of moisture is thin enough, the attraction can be so strong that the water is unavailable for plant use.
- Available or Capillary: When the soil's water content increases, the layer of moisture surrounding the soil particle thickens, and the attraction between soil and water decreases. The outer layers will then flow to areas of thinner films, and the access can be used by plants.
- Gravitational: If the water content becomes so great that the attraction from the soil cannot overcome the pull of gravity, the water will move downward through the pore spaces. This moisture is mostly not available to plants, as it will not remain in the root zone for any length of time.
Soil Air
Because of the irregular sizes and shapes of soil particles, spaces or pores between the mineral and organic matter particle. These pores are occupied by water, microorganisms or air. Optimally, the percentage of air should be 25% by volume. (CO2) content is much higher, and oxygen (O2) much lower in soil than that of the atmosphere, in fact CO2in soil is often several hundred times higher than the .03% normally found in the atmosphere.The volume and composition of soil air are determined by the water content of the soil. Air is found in those pores not occupied by water. After a rain or irrigation the large pores are the first to be emptied of soil water, (capillary action), followed by medium size pores as moisture evaporates or is used by plants.
This easily explains the tendency for soils with a high proportion of tiny pores to be poorly aerated. In this type of soil, water dominates, and the air content is low, as is the rate of exchange of air into and out of the soil from the atmosphere. The result is excessive levels of CO2 and deficient levels of O2: conditions unsatisfactory for plant, root, and microbial growth.
Chemical Factors
All matter is made up of one or more elements. An element is a substance which cannot be broken down into a simpler substance.An element is made up of huge numbers of atoms. An atom is the smallest portion of an element that still retains the properties of the element. Atoms contain three major types of subatomic particles called protons, neutrons and electrons. Protons and electrons are electrically charged. The positively (+) charged protons and the negatively (−) charged electrons are attracted to each other by their opposite charges. Neutrons are neutral, and have no electrical charge. The center or nucleus of an atom is made up of protons and neutrons. Electrons, attracted to the positively charged nucleus, orbit around it. If you could isolate a single atom, it would have the same number of protons and electrons; therefore it would be electrically neutral. But atoms bump into each other all the time getting some of their electrons knocked out of orbit, or stealing them from each other. When this happens the atom no longer has equal number of protons and electrons: it is then positively or negatively charged. In this state the atom is called an Ion. A Cation (+) is a positively charged Ion, and an Anion (−) is a negatively charged Ion.
Most chemical interactions occur between Ions: their positive or negative charges determine their behavior in the soil system. An Ion might be a single element such as calcium (CA2), or molecule such as Nitrate(NO3). The superscript indicates the Ion's oxidation state, and show how many electrons it must give up, (if it is a Cation), or accept, (if it is an Anion), to form a stable compound with a neutral charge. Some elements, like Iron (Fe) might be present in different oxidation states: Fe2+ or Fe3+.
CATION EXCHANGE CAPACITY (CEC)
The Cation exchange capacity, (CEC), is a major measure of potential for the soil's inherent fertility. The CEC indicates the soil's ability to store Cat ions, which include some of the major plant nutrients, (K+).
The CEC depends mostly on a soil's colloidal content. Colloidal in soil are gelatinous substances made of clay particles, raw organic matter, an humus. They have a large surface area relative to their weight, and many negatively charged Anion sites. The positively charged Cations are held in place at these exchange sites, (opposite charges attract one another), safe from leaching but available to the plant roots.
Cation exchange capacities are expressed in milliequivalents, (mEq), (how many thousandths of a gram of hydrogen, or its equivalent charge, can be held by 100 grams of dry soil). The higher the soil's CEC, the greater the quantity of mineral nutrients needed to fill its reserves, but the longer they will be held available in the soil. CEC's in western soils generally range between 10 to 30 mEq, where as a predominantly sandy soil will have a CEC of below 4 mEq. A soil with a low CEC can be significantly improved by adding more humus. Pure humus has a CEC value of about 100 mEq per 100 grams.
Base Saturation Ratio
The major Cations (+) nutrients are calcium, magnesium, potassium and sodium. To assure adequate plant nutrition they must be present in certain minimum amounts, which must also be balanced. The excess of any one mineral, for example magnesium, may interfere with the availability of another such as potassium, even if the lab tests show adequate potassium levels.Base saturation refers to the percentage of a soil's CEC occupied by the base elements, (Cations other than hydrogen or aluminum).
Some efforts have been made to find the "ideal" Cation balance or base saturation ratio: They are : potassium 3–5%, magnesium 10–15%, calcium 65–75%.
In most cases it is not necessary to completely saturate the exchange complex, (exchangeable sites), with these exchangeable base elements. Usually an 80% to 90% saturation of the CEC with a balanced ration of exchangeable bases will be adequate for high yields of most crops.
| A list of Elements Commonly Found in Plants | |||||
|---|---|---|---|---|---|
| Element | Symbol | Element | Symbol | Element | Symbol |
| Barium | Ba | Hydrogen | H | Oxygen | O |
| Boron | B | Iodine | I | Phosphorus | P |
| Bromine | Br | Iron | Fe | Potassium | K |
| Calcium | Ca | Magnesium | Mg | Selenium | Se |
| Carbon | C | Manganese | Mn | Sodium | Na |
| Chlorine | Cl | Molybdenum | Mo | Sulfur | S |
| Cobalt | Co | Nickel | Ni | Vanadium | V |
| Copper | Cu | Nitrogen | N | Zinc | Z |
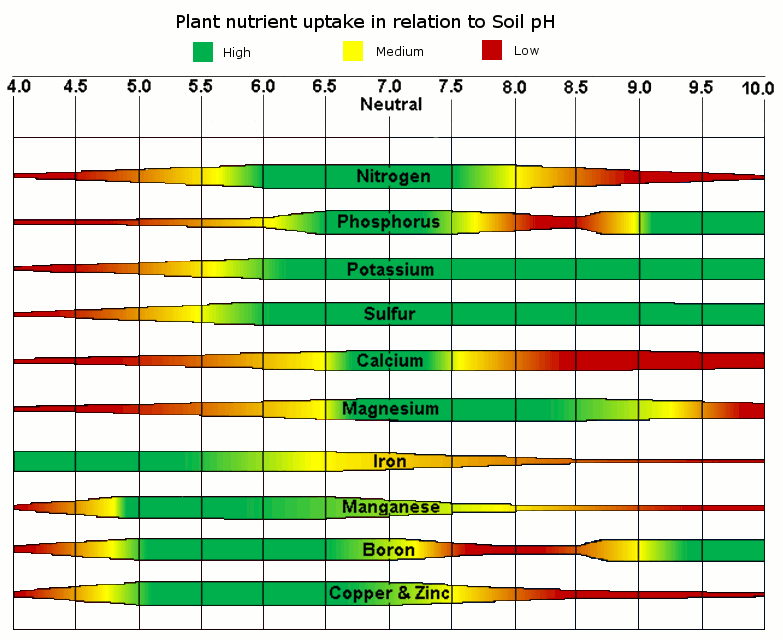
Soil pH
pH is a measure of a substance's acidity or alkalinity, determined by the concentration of hydrogen Ions in a water or salt solution.
Acid soils are low in fertility because too much of the Cation exchange capacity is occupied by either hydrogen, which is not a plant nutrient or aluminum, which is toxic to plants. No more than 12% of the soil's CEC should be occupied by hydrogen Ions. Addition of calcium ( a Cation) to soil substitutes for and replaces the excess hydrogen on the exchangeable sites, neutralizing acids in the soil.
| A pH Scale | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Acid) | (Neutral) | (Alkaline) | ||||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| Vinegar | H2O | Ammonia | ||||||||||||
Some hydrogen Ions are necessary to make most nutrients available with the ideal pH being at 6.4 with 12% hydrogen on the exchangeable sites.
Many of the soil microorganisms involved in making nutrients available to plants are more active at 6.4 pH. At 5.6 pH, nearly all antibiotic production by soil bacteria stops rendering the plant's root system susceptible to fungi pathogen attack.
Some problems associated with acid soils below 6.4 pH:- Interference with the availability of nutrients to plants.
- Increased solubility to Fe, Mn, Zn, Cu, and especially aluninum to undesirable levels.
- Reduced baterial activity, especially the antibiotic production and nitrogen – fixing rhizobia, and slower release nutrients from organic matter.
- Lower total CEC, which further increases the leachability of nutrients.
Alkaline soils are even more troublesome because they are more difficult to correct. The addition of acid forming materials like sulfur, is expensive and only a temporary correction. Usually, alkaline soils reflect over fertilization, wet soils or naturally salty soils.
Some problems associated with alkaline soils above 6.4 pH:- Unavailability of many nutrients, and especially micronutrients.
- Saline seep, causing soil to crust, leading to low soil oxygen levels and increased anaerobic bacteria populations. This condition increases microbial fermentation of organic materials which results in toxic levels of alcohol products.
- Toxic levels of sodium, selenium and other minerals
- Chemical destruction of organic matter
Soil Anions
The major plant nutrients that occur mainly as negatively charged Anions are carbon, nitrogen, phosphorus and sulfur. These nutrients form weak acids in water. They are held in soil reserves in the form of complex organic compounds, unlike Cations, which bond chemically to soil colloids.When added to the soil as soluble fertilizers, Anion nutrients may be lost because they volatilize into the atmosphere, leach away or revert to more stable insoluble forms. These soluble fertilizers may be acid forming and harmful to soil organisms. When substituted for nutrient sources right in organic matter, they can become an addiction; higher doses will continually be required to replace the nutrients that were previously supplied by the soil's ecosystem.
| Some Cations | Some Anions |
| K | C |
| Mg | N |
| Ca | P |
| Na | S |
BIOLOGICAL ASPECTS OF THE SOIL SYSTEM
The cycles that permit nutrients to flow from soil to plant are all interdependent, and proceed only with the help of the soil community. Soil microorganisms are the key link between mineral resources and plant growth.
Soils contain five major groups of microorganisms: Bacteria, actinomycetes, fungi, algae and protozoa. The bacteria are most prominent because of the many different populations in a given soil, and the fact that they are the most abundant group, usually more numerous that the other four combined.
Although many changes similar to those of bacteria are carried out by the other groups, the bacteria stand out because of their capacity for rapid growth and fast decomposition of a variety of natural materials.
Bacteria from soil can be placed in two broad divisions: The indigenous species that are true native residents and the invaders.
Indigenous types may have resistant stages and endure for long periods without being metabolically active, but at the same time these natives multiply and participate in the biochemical functions of the community.
In contrast invader species do not participate in any important way in community activities. They enter the soil system with rain, disease tissues, animal waste or sewage sludge. They may persist for some time in a resting state, and sometimes even grow for short periods; but never do they contribute measurable to the various ecologically significant transformations or interactions.
Bacteria flourish dramatically when readily available nutrients are added to the soil. These actively metabolizing bacteria need nutrients provided from outside sources for their rapid growth, but the supply is quickly exhausted. In other words, bacteria respond promptly to soil amendments, become and remain numerous as long as the nutrients are available, then decline once their food source is depleted.
Environmental conditions affect the density and composition of bacterial flora. The primary environmental variables that influence soil bacteria include: moisture, aeration, temperature, organic matter, pH and inorganic nutrient supply.
Highly acidic or alkaline conditions inhibit many common bacteria; the optimum pH range for most species is about 6.4 to 6.8. Generally, the greater the hydrogen Ion concentrations are the higher the acidity will be and the smaller the size of the bacterial community.
Bacteria and fungi play an essential role in decomposition, but they are also crucial to life on Earth because of their exclusive ability to perform key biochemical changes:
- Nitrification: Changes ammonium, a product of decomposition, into a form of nitrate used by plants.
- Sulfur oxidation: Sulfur is oxidized and made usable to plants through the sulfur cycle, which is similar to the nitrification cycle.
- Nitrogen fixation: Introduces atmospheric nitrogen into the food chain.
- Mycorrhizal association: Fungal penetration of plant roots increases nutrient, (mainly phosphorus), uptake.
- Filaments of Microbial tissue form a network around and through mineral particles in the soil.
- Certain soil organisms produce polysaccharides, (complex sugars), that have a mucilaginous nature and cement mineral particles together. This results in up to 40% improved erosion stability in some soils.
- Other non-polysaccharide microbial produced binders for reducing erosion and leaching include lignin-like and humic substances.
- Deposition of organic matter that strengthens clay particle bonding and assists in aggregation soil particles into water soluble units.
The benefits of this soil structuring also include improved plant root growth, adequate aeration and improved nutrient holding ability.
Microbial transformation of elements such as nitrogen, carbon, phosphorus, sulfur, iron, potassium, manganese, selenium, zinc and copper occur in the soil system on a regular basis.
THE NUTRITIONAL NIGHTMARE
Bacteria and fungi control diseases of plants and soils:- Suppression (Production fo Antibiotics)
- Competition (Striving for the same object)
- Antagonism (Active opposition)
- Predator (Plunders or kills)
When man interferes with these natural functions, he creates a biological nightmare that he and his plants must become slaves to, unless he chooses to wake up from his bad dream.
The natural healthy state for a grass plant is to let it grow to its full, reproductive state. On the golf course, however, man intervenes and mows the grass to 3/16" in height on a weekly basis causing open, damaged cells, excessive need for nutrients, and debilitating energy loss to the plant.
Then man exerts a great crushing pressure on the delicate cell walls of the composite leaf, causing cell wall and vascular tissue injury. With the same "spiked grass crusher", man proceeds without concern for sanitary hygiene of the plant, by not using food condoms, to most efficiently transfer disease inoculums from one grass ecosystem (greens) to another.
As the injured and infected patient tries to deal with the tough rigors of day-to-day survival the best it knows how, its caretaker decides its lunchtime. For the first course, a hearty vegetable soup of broth (liquid N) salt (K) and pepper (P) but there seems to be something missing. Where is the substance? Meat (Ca & Mg), potatoes (S), carrots (Zn & Mn), green beans (B & Cu), onions(Se & Mo) and corn (Co, V & Ni); these are not included in this diet. The caretaker has just piled enough addicting, substance-less junk food around the grass plants to last for two months.
The patient, now a drug addict, is rushed to emergency, suffering from a drug overdose. Infection (anthracnose and fusarium) sets in and mega doses of drugs (fungicides) are administered to the weak and dying patient. Now in its immune compromised state, the doctor (grounds superintendent) prescribes more vegetables soup (broth, salt and pepper). The patient's lab tests indicate anemia so a STAT blood transfusion (iron) is ordered. As the infection becomes critical, more drugs (fungicides) are prescribed. A skin carcinoma (dead brown patches) develops and begins to spread. A transplant is ordered by the doctor, but too late; the patient dies of addiction and drug overdose.
Meanwhile the disease contaminated body fluids were spread by man's feet to other once healthy greens and an epidemic begins among the rest of the immune compromised golf course residents.
This nightmare plagues not only the golf course industry; it is equally frightening to the agricultural industry. Is there a way out of this bad dream? Yes! "Balanced Nutrition".
SOIL AND PLANT MICROBIAL SAMPLING
- Obtianing a Soil Sample
a bucket mix well and air dry for several hours. Place approximately 1 cup of this soil in a A & L Midwest Lab bag, and fill out the necessary form. For a normal soil test report used in our Bio System program, check items 1A, 2, 3 and NO3N on the sample form (See example, next page). If an anaerobic and aerobic bacterial plate count is needed, simply write it across the face of the form. - Obtaining a Tissue Sample
For tissue tests, we perfer to use quite a different approach than others use. - For annual crop plants up to 6" tall
Send entire plants from 15 different field locations. - For annual crop plants over 6" tall
Sample the bottom leaf closest to the ground, then the leaves from half way up the plant, then a sample from a new growth tissue. As with the smaller plants, you will need these samples from 15 different plants from random field lacations.
It is our belief that a petiole test on any plant tissue test is not a correct reflection of the nutrition present in the plant leaves. We do not believe that selecting leaves from only one location, (i.e. 3rd leaf from the top), is an acceptable method either.
Plant disease usually begins on the lower leaf tissue. In light of the fact that certain elements are mobile and always drown to the new tissue or reproductive organs, it only makes sense to have a sample representing the whole plant rather than just the new or middle aged tissue. Quite frequently the new growth will not reflect true overall plant tissue deficiencies.
On perennial crops, trees and shrubs, a sample leaf from an older branch, and one leaf from the new tissue area should be combined from 15 representative plants.
On golf greens a mower-obtained sample will suffice; but on fair way grasses the sample should represent as much of the whole plant as possible.
Avoid tissue in all cases from excessively dusty leaves; or rinse leaf tissue sample in water prior to drying. If next day or second day shipment is made, drying the sample is not necessary. For conventional shipping, dry the tissue before placing in Midwest Labs tissue sample bag. Fill out tissue from, indicating complete analysis.
Copyright 2013 © Modern Ag Products LLC
